A recent review by Momoli, Costa, Lenti, Tubertini, Parenti, Martella, Varchi, and Ferroni, published in Cancers 2025, explores the transformative potential of advanced three-dimensional (3D) in vitro models combined with Machine Learning (ML) and Artificial Intelligence (AI) in anticancer research.
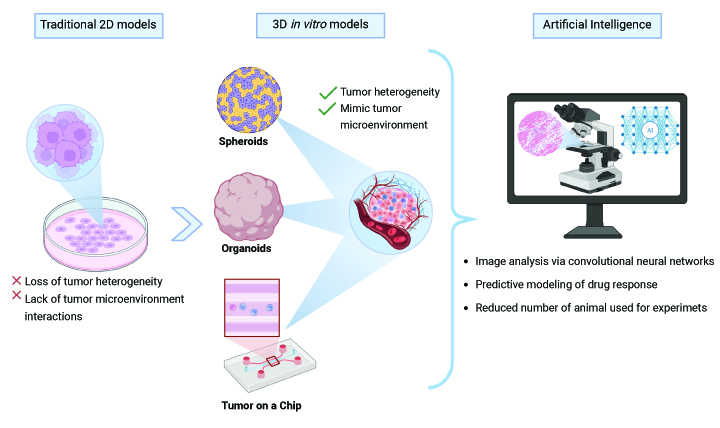
This work highlights the evolution of 3D in vitro models, such as spheroids, organoids, and tumor-on-a-chip (ToC) systems, which offer a more accurate representation of the tumor microenvironment (TME) compared to conventional two-dimensional (2D) cultures. These advanced models enable a deeper understanding of tumor biology, progression, and therapeutic responses. The integration of ML and AI further enhances these models by improving predictive accuracy, facilitating complex data analysis, and optimizing experimental conditions. For instance, ML models can analyze images from 3D cultures to extract features like cell motility and spheroid sensitivity to drugs, reducing time and cost. Furthermore, ML frameworks can integrate diverse datasets, including genomic and transcriptomic profiles, to predict drug responses with higher accuracy.
The synergy between 3D in vitro models and AI-driven analytics is poised to revolutionize anticancer drug development. These technologies offer a robust platform for high-throughput drug screening, optimization of treatment regimens, and the identification of novel therapeutic targets. Crucially, this approach significantly reduces the reliance on animal testing, aligning with the “three Rs” principles (replacement, reduction, and refinement) for ethical research. By providing more physiologically relevant and human-specific data, these advancements pave the way for more efficient, targeted, and personalized cancer therapies, ultimately improving patient outcomes.
The full review is available in Cancers 2025 via the provided DOI: